The Science Behind Cleaning Agents: How Different Ingredients Work at a Molecular Level
Cleaning is an everyday task, yet not many of us ponder the science at play when we scrub a surface or launder our clothes. At the heart of every cleaning process are cleaning agents, and understanding them from a molecular perspective is both fascinating and enlightening.
[Placeholder for Image 1: A molecular structure of a common cleaning agent]
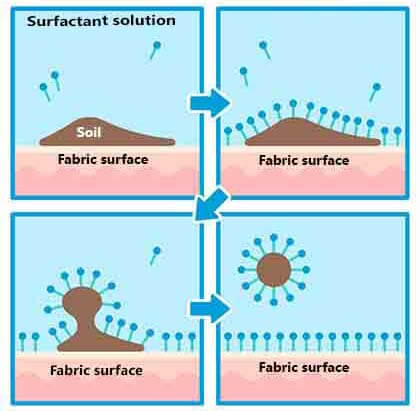
1. The Power of Surfactants
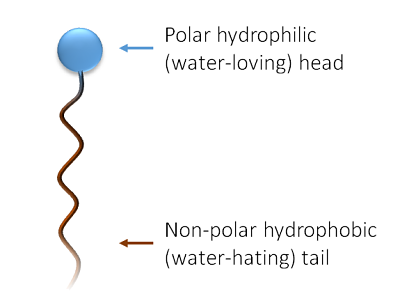
Surfactants, or surface-active agents, are the most common type of cleaning agents. They reduce the surface tension of water, allowing it to better interact with oil and grease.
- How they work: Surfactant molecules have a hydrophilic (water-attracting) head and a hydrophobic (water-repelling) tail. When used in cleaning, the hydrophobic tails bind to oil and grease while the hydrophilic heads remain in the water. This action encapsulates the dirt and lifts it off the surface.
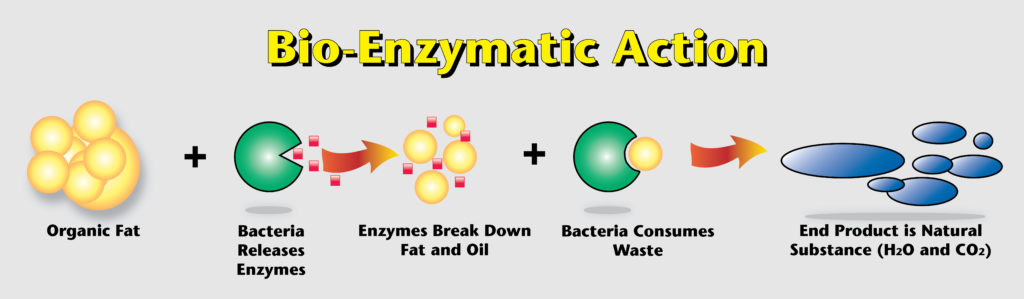
2. Enzymes in Cleaning
Enzymatic cleaning agents contain specific enzymes that target particular types of soil. For instance, proteases break down protein-based stains, while lipases tackle fat-based stains.
- How they work: Enzymes speed up the chemical reactions that break down stains, making them easier to remove from surfaces. They essentially “digest” the stains.
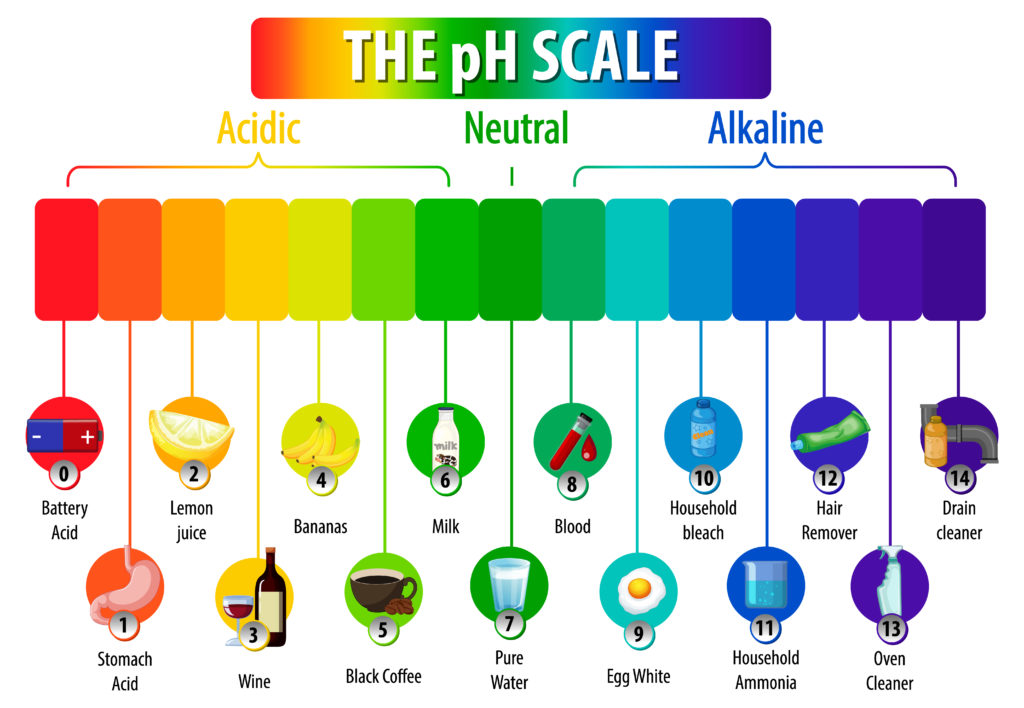
3. Acids and Bases
Many cleaning agents are either acidic (like vinegar) or basic (like baking soda). Their pH level determines their cleaning ability.
- How they work: Acids release hydrogen ions which can break down mineral deposits like lime and rust. Bases release hydroxide ions that can saponify fats, turning them into soap and glycerol, thus cleaning the surface.
[ pH scale showing where common household cleaners fall]
4. Oxidizing Agents
Oxidizing agents like bleach or hydrogen peroxide work by releasing oxygen. This oxygen can break the chemical bonds of a stain or kill bacteria.
- How they work: On a molecular level, the released oxygen can break apart chromophores – the part of a molecule responsible for its color, thereby “bleaching” the stain.
[Placeholder for Image 5: A before-and-after comparison of a stain treated with an oxidizing agent]
5. Polymers
Certain cleaning products, especially in floor finishes or fabric treatments, use polymers to create a protective barrier or sheen.
- How they work: Polymers create a microscopic layer on surfaces, preventing dirt and stains from adhering or providing a glossy finish.
[Placeholder for Image 6: A magnified view showing a polymer barrier on a surface]
In conclusion, understanding the molecular action behind cleaning agents can help in making informed choices about the products we use. It’s a blend of chemistry and purpose, designed to keep our environments clean and hygienic.

